This electron transfer creates positive metal ions cations and negative nonmetal ions anions which are attracted to each other through coulombic attraction.
Ceramics are formed by what type of bond.
For metals the chemical bond is called the metallic bond.
The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic.
Recall that the predominant bonding for ceramic materials is ionic bonding.
Covalent bonding is found in many ceramic structures such as sic bn and diamond.
The high energy of covalent bonds makes these ceramics very stable with regard to chemical and thermal changes.
Underlying many of the properties found in ceramics are the strong primary bonds that hold the atoms together and form the ceramic material.
Many ceramic materials have covalent bonds.
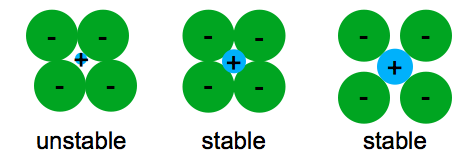
The ionic bond occurs between a metal and a nonmetal in other words two elements with very different electronegativity.
In ionic bonding a metal atom donates electrons and a nonmetal atom accepts electrons.
The atoms in ceramic materials are held together by a chemical bond.
They are either ionic in character involving a transfer of bonding electrons from electropositive atoms to electronegative atoms or they are covalent in character involving orbital sharing of electrons between the constituent atoms or ions.
The primary difference between ceramics and other materials is the chemical bonds that hold these materials together.
Compounds with covalent bonds may be solid liquid or gas at room temperature depending on the number of atoms in the compound.
Ceramics can vary in opacity from very translucent to very opaque.
A material held together by either type of bond will tend to fracture before any plastic deformation takes place which results in poor toughness in these materials.
According to this definition elemental carbon is a ceramic.
These chemical bonds are of two types.
Additionally carbon based materials such as carbon fiber carbon nanotubes and graphene can be considered ceramics.
The two most common chemical bonds for ceramic materials are covalent and ionic.
Noncrystalline the more translucent it will appear and the more crystalline the more opaque.
In general the more glassy the microstructure i e.
Two types of bonds are found in ceramics.
This makes many solid materials with covalent bonds brittle.
Ceramic materials are usually ionic or covalent bonded materials and can be crystalline or amorphous.
A common definition of a ceramic is a hard material that is held together with ionic and covalent bonds.
Although they can contain covalent bonds such as the si o si linkages in glass they are often characterized by ionic bonds between positive and negative ions.
In each molecule the bonds between the atoms are strong but the bonds between molecules are usually weak.
The atoms in these ceramics are arranged so that each pair of nearest neighbour atoms forms a chemical bond by sharing a pair of electrons.